Clinical Pathology Laboratories has validated the Roche cobas® HIV-1/HIV-2 Qualitative test for Serum or EDTA Plasma. This is a Real-Time Polymerase Chain Reaction (PCR) test for use with the cobas® 6800/8800 platform and is approved by the Food and Drug Administration (FDA) for use as an aid in the diagnosis of HIV-1/HIV-2 infection. The cobas® HIV-1/HIV-2 Qualitative test may be used to confirm the presence of HIV-1 or HIV-2 infection in an individual with specimens reactive for HIV-1 or HIV-2 antibodies or antigens. Additionally, the presence of HIV-1 or HIV-2 nucleic acid in the plasma or serum of individuals without antibodies to HIV-1 or HIV-2 is indicative of acute or primary infection. The assay may also be used as an aid in the diagnosis of infection with HIV-1 and/or HIV-2 in pediatric subjects and pregnant women.1
Effective February 26, 2024, CPL will implement the cobas® HIV-1/HIV-2 Qualitative test in the following order codes to streamline HIV diagnosis and accommodate different patient indications:
|
General Screening, Prenatal Screening |
3540 |
HIV Ag/Ab Assay (4th gen), Reflex Confirmation |
|
Acute Infection, Neonatal Screening |
3548 |
HIV-1/HIV-2 Qualitative NAAT |
|
Acute Infection, Neonatal Screening |
3566 |
HIV-1/HIV-2 Qualitative Reflex Quantitative |
|
Clinical Indication |
Order Code |
Test Name |
|---|
The Centers for Disease Control and Prevention (CDC) has a published recommendation for an updated HIV diagnostic algorithm with provisions for recently FDA-approved HIV-1/HIV-2 Nucleic Acid Amplification Tests (NAAT) with a diagnostic claim.
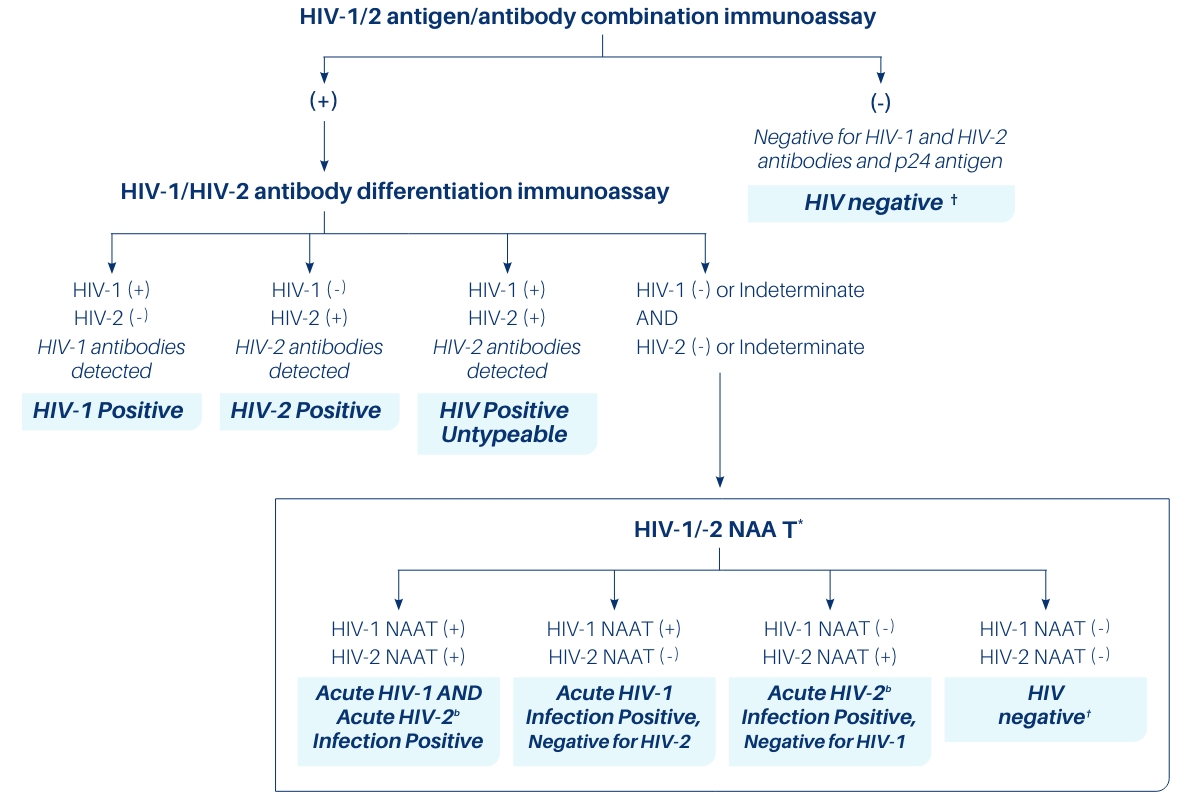
*NAATs that have a diagnostic claim
b Data on interpreting acute HIV-2 infection is limited
† Consider individual’s history in deciding whether follow-up testing is warranted
See the updated guidelines at2
https://www.cdc.gov/hiv/guidelines/recommendations/technical-update-for-hiv.html
Effective February 26, 2024, CPL will add the HIV1/2 Qualitative NAAT as a third reflex test to the current HIV screening order code 3540 HIV Ag/Ab Assay (4th gen), Reflex Confirmation. All tests in the HIV screening algorithm can be run on the same specimen. This includes the recommended update to the CDC guidelines. Benefits of HIV1/2 Qualitative NAAT as a third reflex test for HIV screening include:
- Greatly reduced Inconclusive final HIV screening results.
- Greatly reduced need to redraw plasma for HIV screening confirmation.
- Ability to differentiate HIV-1 and HIV-2 in a single highly specific and sensitive test.
HIV1/2 Qualitative NAAT will also be offered as a single test for Serum or Plasma and with reflex to the appropriate viral load for Plasma only.
For HIV Ag/Ab (4th gen), Reflex Confirmation (order code 3540), CPL has reduced room temperature and refrigerated stability requirements and increased required volume to ensure all specimens will meet the requirements for the NAAT as a possible third reflexed test.
|
3540 |
HIV Ag/Ab Assay (4th gen), Reflex Confirmation |
Serum |
3mL |
2mL |
7 Days Room Temp 4 Weeks Refrigerated 3 Months Frozen |
2 Days Room Temp 7 Days Refrigerated 6 Weeks Frozen |
|
3548 |
HIV-1/HIV-2 Qualitative NAAT |
Serum or Plasma |
3mL |
2mL |
N/A |
2 Days Room Temp 7 Days Refrigerated 6 Weeks Frozen |
|
3566 |
HIV-1/HIV-2 Qualitative Reflex Quantitative |
Plasma |
3mL |
2mL |
N/A |
2 Days Room Temp 7 Days Refrigerated 6 Weeks Frozen |
|
Order Code |
Name |
Specimen |
Preferred |
Minimum |
Previous Stability |
New Stability |
|---|
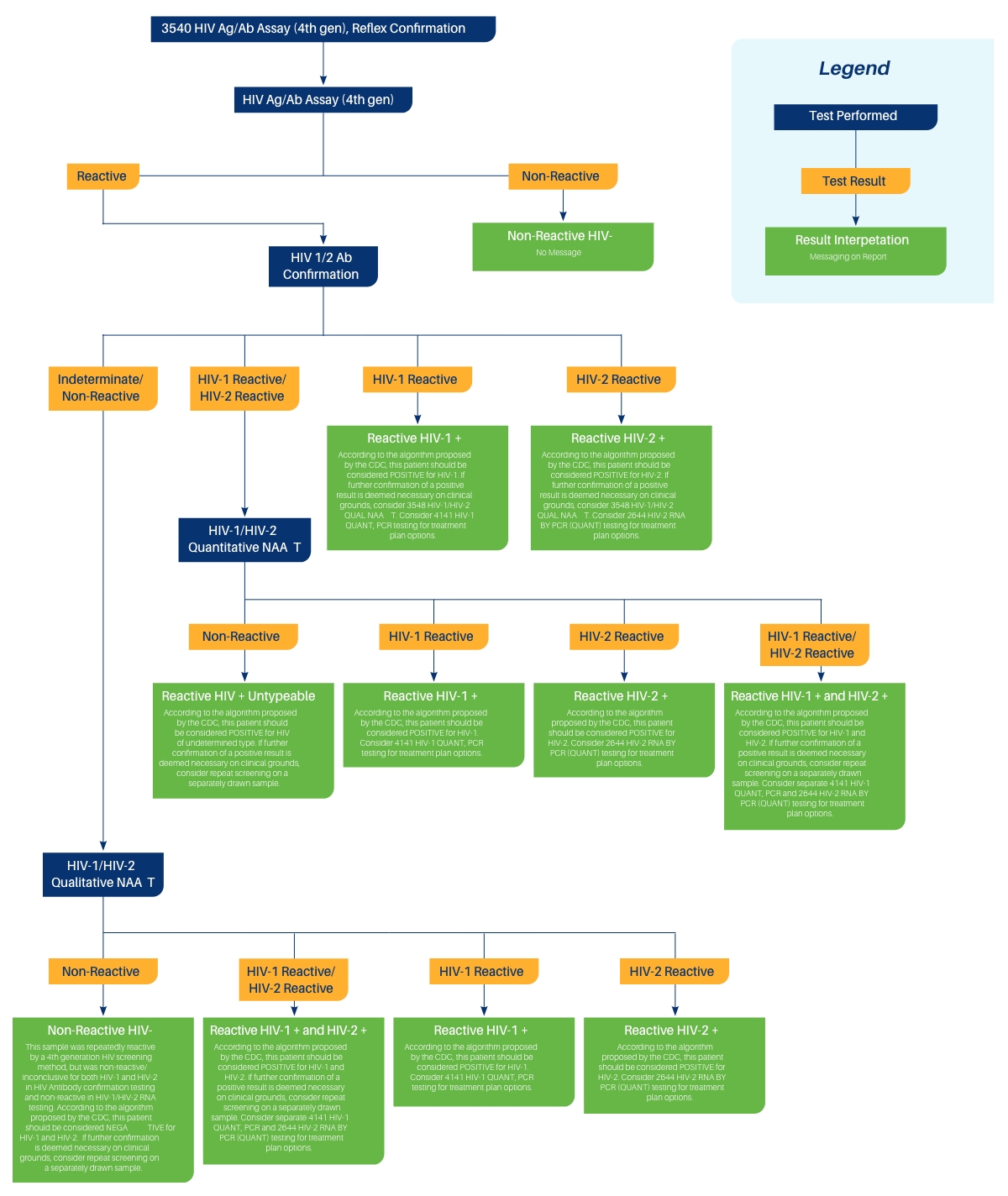
CPL has updated the interpretive comments used with HIV Ag/Ab Assay (4th gen), Reflex Confirmation (order code 3540) to reflect the addition of the HIV-1/HIV-2 Qualitative NAAT as the third reflexed test. See the following flowchart.
References
- cobas HIV-1/HIV-2 Qualitative. Instructions for Use. 09198695001-04EN rev, 4.0.
- https://www.cdc.gov/hiv/guidelines/recommendations/technical-update-for-hiv.html
Last reviewed May 16, 2023
