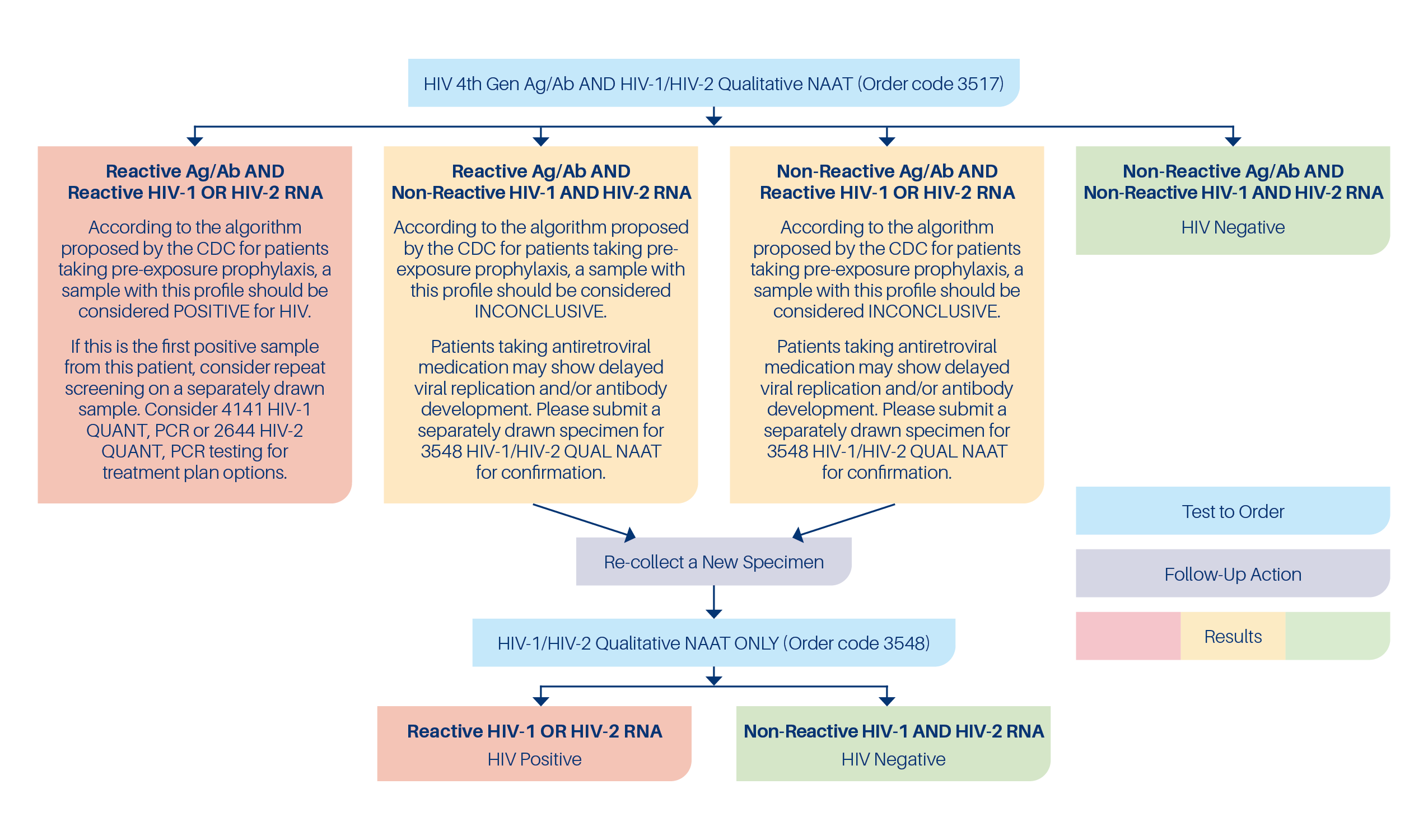
Effective May 13, 2024, CPL is pleased to announce the introduction of an HIV diagnosis panel for use with patients taking antiretroviral medication for pre-exposure prophylaxis (PrEP). The United States Centers for Disease Control and Prevention (CDC) has released clinical practice guidelines for use of PrEP https://www.cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf.The CDC guidelines include a separate algorithm for HIV screening specific for the periodic testing of patients using PrEP. The screening algorithm for patients on PrEP accounts for the observed delay in viral replication and antibody development caused by the antiretroviral medications used for PrEP. The CPL HIV PrEP screening panel uses the algorithm promoted in the CDC guidelines with concurrent HIV 4th Generation Ag/Ab and HIV-1 and HIV-2 Qualitative NAAT testing. If initial specimen is inconclusive, a second specimen is recommended for HIV-1 and HIV-2 Qualitative NAAT only. See algorithm below.
Serum specimens only are acceptable for HIV PrEP panel testing.
| 3517 | HIV PrEP Panel |
HIV Ag/Ab | Non-Reactive | 2 mL Serum | 87389 |
2 Days Room Temperature 7 Days Refrigerated 6 Weeks Frozen |
| HIV-1 RNA | Non-Reactive | 87535 | ||||
| HIV-2 RNA | Non-Reactive | 87538 | ||||
| Order Code |
Test Name |
Reportable Results |
Reference Range |
Minimum Volume |
CPT Codes |
Stability |
|---|
This test is only intended for HIV screening on patients with recent or ongoing antiretroviral pre-exposure prophylaxis. For general screening consider 3540 HIV 4th Gen reflex Confirmation.
References
Preexposure Prophylaxis for the Prevention of HIV Infection in the United States – 2021 Update Clinical Practice Guideline
