Offering our clients state-of-the-art testing is part of CPL’s ongoing commitment to excellence.
Effective February 27, 2024, Clinical Pathology Laboratories (CPL) will update the rejection criteria for C. difficile Toxin A/B with C. difficile GDH Reflex Toxin/PCR to include formed stools. As a reminder, the reflex testing follows the recommendations of the Infectious Diseases Society of America (IDSA) and the Society for Healthcare Epidemiology of America (SHEA). The algorithm offers a sensitive, specific, and cost-effective approach for the diagnosis and treatment of C. difficile.
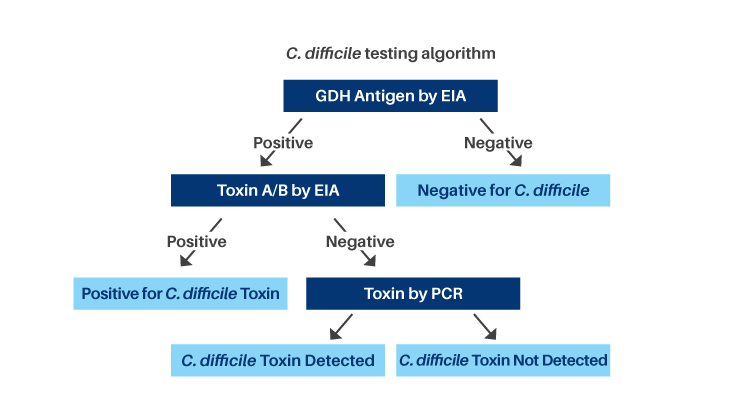
The reflex algorithm includes:
- C. difficile Glutamate Dehydrogenase (GDH) Antigen, an Enzyme Immunoassay (EIA), is used as a screening test to detect C. difficile antigen. GDH Antigen is a metabolic enzyme produced in high amounts by toxigenic and nontoxigenic strains of C. difficile
- GDH negative results indicate the sample is negative for C. difficile
- GDH positive results indicate the presence of C. difficile but do not specifically indicate toxin production
- Toxin A and B by EIA is performed to detect toxigenic C. difficile
- Toxin positive results indicate the sample is positive for toxigenic C. difficile
- Toxin negative results require discriminatory testing by Polymerase Chain Reaction (PCR) as the toxin may degrade in stool specimens. Approximately 8% of specimens will require PCR testing
Note: Reflex testing is performed at additional charge(s) and CPT code(s)
Sensitivity and Specificity for C. difficile testing
| Toxin A/B by EIA | 32-99% | 84-100% |
| Toxin by PCR | 73-100% | 91-100% |
| GDH Antigen + Toxin A/B by EIA | 41-92% | 94-100% |
| GDH + Toxin A/B by EIA + Toxin by PCR | 68-100% | 97-100% |
| C. difficile Testing Methodology | Sensitivity | Specificity |
|---|
C. difficile GDH Reflex Toxin/PCR
| Order Unit Code/Test Name: | 6335 - C. difficile GDH Reflex Toxin/PCR |
| Test Method: | Enzyme Immunoassay (EIA) |
| Specimen Requirements: | 10 mL fresh stool. Refrigerate. If specimen cannot be delivered to lab within 72 hours, freeze. |
| Specimen Rejection Criteria: | Will reject if:
|
| Transport Temperature: | Refrigerated (2-8 °C) |
| Stability (collection to initiation of testing): | Room Temperature Unacceptable Refrigerated (2-8 °C) 3 Days Frozen (≤ -20 °C) 1 Month |
| Performed: | Sunday-Saturday AM Shift |
| Analytic Time: | 1 Day Note: Analytic time may be longer if PCR testing is required |
| Reference Range: | Negative |
| Interpretive Information: |
|
| CPT Code: | GDH - 87449 Toxin A and B - 87324 Toxin PCR - 87493 |
References:
Clinical Practice Guidelines for Clostridium difficile Infection in Adults: 2010 Update by the Society for Healthcare Epidemiology of America (SHEA) and the
1. Infectious Diseases Society of America (IDSA). https://www.cdc.gov/HAI/pdfs/cdiff/Cohen-IDSA-SHEA-CDI-guidelines-2010.pdf
2. Laboratory Diagnosis of Clostridium difficile Infections: There is Light at the End of the Colon, Brecher et al, Clinical Infectious Diseases, 2013, 57 (8): 1175.
(Summarizes multiple studies)
