As part of our ongoing commitment to improving the diagnosis of kidney disease, CPL is pleased to offer eGFR by creatinine and cystatin C (eGFR-cr-cys). The cystatin C assay is a particle enhanced immunoturbidimetric assay which is FDA-cleared for in vitro quantitative determination of cystatin C in human serum.
In accordance with the NKF-ASN Task Force guidelines, CPL has adopted the 2021 eGFR-creatinine-cystatin C equation for adult patients (age ≥ 18 years). For pediatric patients, NKF hosts a calculator that includes additional biometric information (https://www.kidney.org/professionals/kdoqi/gfr_calculatorped).
Chronic kidney disease (CKD) is a worldwide health problem with significant impact on cardiovascular and overall morbidity and mortality. Glomerular filtration rate (GFR) is critical to provide an accurate diagnosis of CKD as an aid to guide management and predict prognosis. GFR may be directly measured by the clearance of either exogenous or endogenous (e.g., timed creatinine clearance) filtration markers.
For most clinical circumstances, estimating GFR from serum creatinine (eGFR-cr) is appropriate for staging and tracking the progression of CKD. However, the influence of factors including age, muscle mass, diet, and tubular secretion on creatinine may render eGFR-cr inaccurate in some clinical circumstances.
Cystatin C is a low molecular weight cysteine protease inhibitor produced at a constant rate by all nucleated cells, nearly eliminating differences due to muscle mass or gender. Elimination is almost entirely by glomerular filtration, making cystatin C serum concentration inversely correlated with glomerular filtration rate (GFR). Compared with creatinine, several reports have shown that eGFR based on cystatin C is more specific and closely associated with clinical outcomes in CKD. Moreover, the recently published landmark study by the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) demonstrated that new eGFR equations based on both creatinine and cystatin C (eGFR-cr-cys) are more accurate than eGFR equations utilizing creatinine alone.
Based on these findings, the National Kidney Foundation and American Society of Nephrology (NKF-ASN) Task Force recommended national efforts to facilitate routine and timely use of cystatin C to confirm eGFR in adults who are at risk for or have CKD.
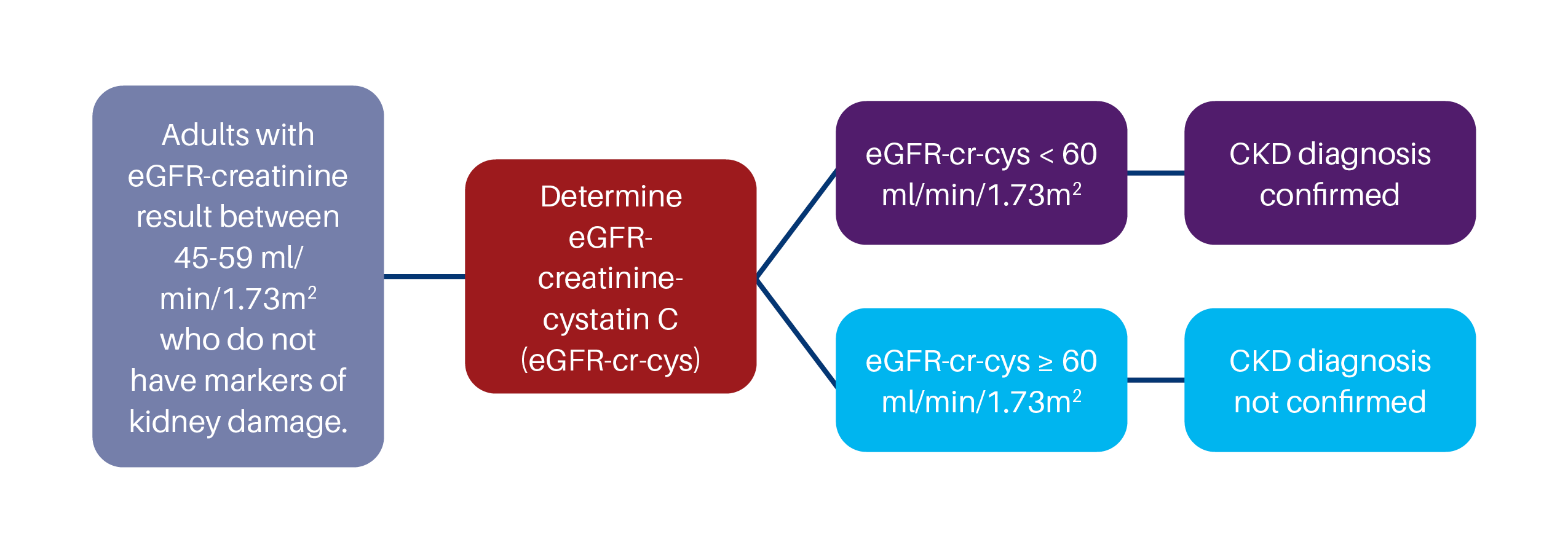
According to Kidney Disease Improving Global Outcomes (KDIGO) practice guidelines, confirmation by eGFR-creatinine-cystatin C is warranted in specific circumstances when decisions depend on more accurate knowledge of GFR. KDIGO provides guidance to confirm CKD as below.
Test Information
| Unit Code | 3057 |
| Test Name | Cystatin C Alias: eGFR with cystatin C-creatinine |
| Component Codes (LOINC) |
Cystatin C: 30571 (LOINC 33863-2) Creatinine: 2214 (LOINC 2160-0) eGFR: 30572 (LOINC 98979-8) |
| Specimen Requirements |
Sample Type: Serum Preferred Container: Serum Separator Tube Alternate Container: Plain Red-Top Tube Sample Volume: 1.0 mL of Serum Transport: Refrigerated Specimen Stability: Ambient: 1 Week, Refrigerated: 1 Week, Frozen: 1 Month |
| Reference Ranges |
Cystatin-C: See Report Creatinine: See Report eGFR (2021 CKD-EPI creat-cystat) >59 ml/min/1.73m2 |
| CPT |
Cystatin-C: 82610 Creatinine: 82565 |
| Unit Code Component | Description |
|---|
References
- Delgado C et al. A Unifying Approach for GFR Estimation: Recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease. Am J Kidney Dis. 2021 Sep 22:S0272-6386 (21) 00828-3.
- Inker LA, et al. New creatinine- and cystatin C–based equations to estimate GFR without race. N Engl J Med. 2021 Sep 23.
- KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013; 3: 1-150.
