Offering our clients state-of-the-art testing is part of CPL’s ongoing commitment to excellence.
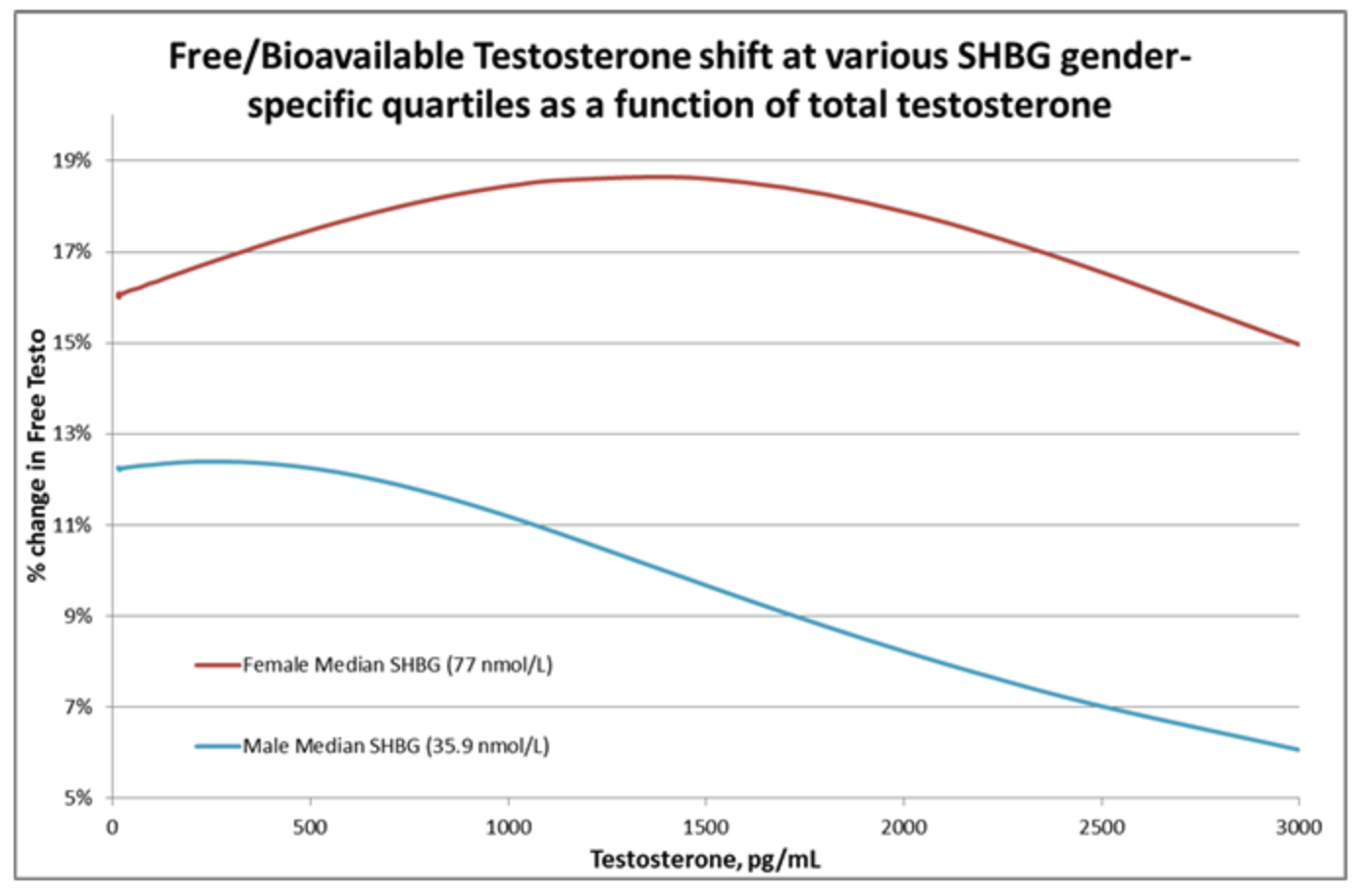
Effective 10/14/2019, a new formulation of Sex Hormone Binding Globulin (SHBG) reagent is implemented by the test manufacturer to correct long-term drift in assay recovery. Validation studies of the new SHBG formulation shows recovery 18% lower than the prior assay. The shift tends to increase the calculated free and bioavailable testosterone an average of 12% for adult males and 16% for adult females. The manufacturer does not provide guidance to adjust reference intervals for SHBG. Additionally, there is no specific recommendation to alter the calculated free or bioavailable testosterone reference ranges. The free and bioavailable testosterone are calculated from total testosterone and adjusted for SHBG and albumin binding according to Vermeulen1, and the reference ranges represent a collaboration with Sonic Reference Laboratory (SRL) who offer LC-MS based measurement of total testosterone, suitable for women and children. The following graph shows the shift in free testosterone as a function of SHBG (prior assay) and total testosterone when calculated using the method of Vermeulen:
 CPL is assessing free and bioavailable testosterone in the patient population to determine suitability of established reference ranges.
CPL is assessing free and bioavailable testosterone in the patient population to determine suitability of established reference ranges.
| Order Codes: | 4933 (SHBG), 4937 (Testosterone, free/total), 4143 (Testosterone, Bioavailable, free and total), 2991 (Transgender hormone profile) |
| Test Method: | Roche COBAS electrochemiluminescent immnoassay |
| Specimen Requirements: | 2 mL serum |
| Transport Temperature / Stability: | Refrigerated, 5 days |
| Performed: | Monday through Friday |
| Analytic Time: | 1 Day |
| Reference Range: | Various |
| CPT Codes: | 84270, 84403 |
1: “A Critical Evaluation of Simple Methods for the Estimation of Free Testosterone in Serum”, Vermeulen, A, Verdonck, L., Kaufman, J.M., Journal of Clinical Endocrinology & Metabolism, 84:10, October 1999, 3666-3672.
