Offering our clients state-of-the-art testing is part of CPL’s ongoing commitment to excellence.
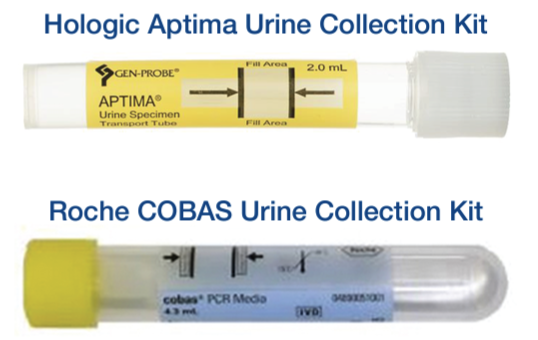
With the ongoing COVID-19 pandemic testing consuming numerous resources, the laboratory industry and its technology vendors are experiencing critical shortages of consumables and reagents. The Hologic Yellow Aptima Urine test system for Chlamydia trachomatis (CT), Neisseria gonorrhea (NG) and Trichomonas vaginalis (TV) is in nationwide backorder, and supplies available to CPL are limited and intermittently resupplied. To supplement collection supplies and reagents, CPL has validated the Roche COBAS 6800/8800 method for CT, NG and TV. The details of the analysis are as follows:
- Roche method is qualitative real-time polymerase chain reaction (PCR)
- Hologic method is target capture and Transcription-Mediated Amplification (TMA)
- The Roche and Hologic methods are both FDA-approved nucleic-acid amplification technologies (NAAT)
- The Roche and Hologic methods are considered as analytically equivalent by CDC
- Fresh urine must be transferred to either the Hologic or Roche tube with fill between the guide marks within 24 hours of void
Given the nature of the test shortage, CPL will substitute Roche PCR for Hologic TMA testing as required to meet appropriate turnaround requirements utilizing the original order codes (unit codes). Affected test codes subject to substitution are given in the chart below:
| Chlamydia and Gonorrhea | 4335 | Profile CT and NG, TMA, Urine |
| Chlamydia trachomatis | 5399 | Chlamydia, TMA, Urine |
| Neisseria gonorrhea | 5397 | Gonorrhea, TMA, Urine |
| Trichomonas vaginalis |
3913 4445 |
Trichomonas, TMA, Urine Trichomonas, Male |
| Organism | Unit Code | Test Description |
Additional notes
- This announcement only applies to urine testing. The SimpleSwab and liquid-based Pap specimens are not affected by this test shortage
- The CT/NG/TV report format is suitable to accept Roche PCR results with comments applied to testing that is redirected as follows:
- Roche: Note: Testing is performed with Roche COBAS 6800/8800 method using real-time polymerase chain reaction (PCR) method
- Hologic: Note: Assay methodology is nucleic acid amplification by transcription mediated amplification (TMA) utilizing the Aptima Combo 2 Assay
- With nationwide test shortages, CDC offers guidance on STI testing to ensure appropriate utilization See URL: www.cdc.gov/std/general/DCL-Diagnostic-Test-Shortage.pdf
We apologize for the inconvenience that this may cause. Please contact your Account Representative should you have any questions. CPL is in regular communication with Hologic as they ramp up production of collection devices and establish additional manufacturing capability.
References:
www.cdc.gov/mmwr/preview/mmwrhtml/rr6302a1.htm
www.cdc.gov/std/general/DCL-Diagnostic-Test-Shortage.pdf
